Abstract
Our current understanding of the effect of BCR::ABL1 oncogene in leukemia development largely relies on studies using in vitro and in vivo leukemogenesis models. Among them, in vivo models using retrovirus-mediated BCR-ABL gene transfer in murine embryonic stem cell (ESC)-derived hematopoietic cells suggested that the enforced expression of BCR::ABL1 was sufficient to confer them an in vivo long-term repopulation potential. However, in these models, the unique BCR::ABL1 dependence of the generation of this potential has not been shown. Our study aimed at getting a better understanding of the early events regulated by BCR::ABL1 during the course of ESC differentiation without retroviral-mediated gene transfer. The elucidation of these early events could also be of major interest in therapy as generation of bona fide HSCs from normal ESC or iPSC remains a major challenge. To this end, we engineered a unique Tet-ON inducible system in which BCR::ABL1(p210) expression level can be tightly controlled using doxycycline (Dox). As compared to previously reported ESC-derived models, our system contains a single copy of BCR::ABL1 inserted into the murine Col1a1 locus under the control of Tetracycline operator, is sensitive to Dox, leakproof and reversible.
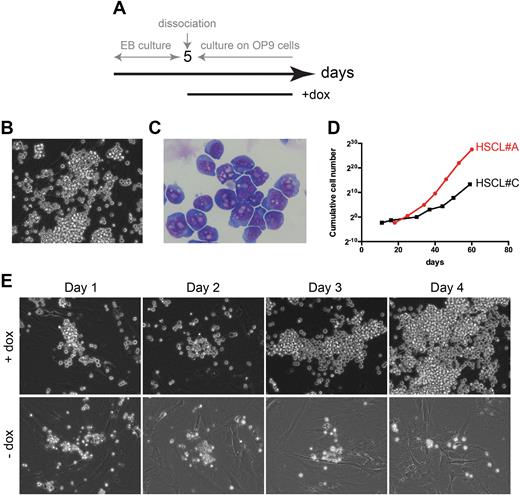
The first striking results was the timing of Dox-inducible BCR-ABL expression during the course of ESC differentiation, impacting both the frequency and emergence of hematopoietic progenitors. Indeed, the expression of BCR::ABL1 at day+5 favors significantly the emergence and maintenance of immature hematopoietic progenitors. Importantly, these cells can self-renew only in the presence of Dox. Several hematopoietic stem-like cell lines were established and two of them (HSCL-A and HSCL-C) have been explored in detail. A major BCR-ABL-dependent proliferative ability was documented in both (Figure 1D). Both expressed Lin-Sca-1+c-Kit+CD150+CD48- and eSLAM, corresponding to phenotypically identified adult mouse HSCs. Importantly, these cells exhibited a normal myeloid and erythroid differentiation capacity in CFC assays, giving rise also to CFU-GEMM. We then evaluated the ability of HSCL to self-renew using long-term culture-initiating cell (LTC-IC) assays. To this purpose, EPCR+CD45+/loCD48-CD150+ (ESLAM) cells were sorted at a single cell level and co-cultured on a supportive AFT024 feeder layers for 4 weeks. LTC-IC-derived progenitors were then assessed using CFC assays. The frequency of ESLAM LTC-ICs was 0.53 from adult bone marrow and 0.52 from HSCL lines demonstrating their self-renewal ability. To better characterize HSCL cell lines, we sorted LSK cell population from HSCL#A, HSCL#C, E14.5 fetal liver and murine adult bone marrow and analyzed their transcriptome signatures by RNA-Seq. Unsupervised principal component analysis with the 1333 genes exhibiting the highest variability revealed that HSCL cell lines clustered together and exhibited distinct molecular signature from fetal and adult LSK. This stratification was validated by clustering with the 50 best variable genes. Among them, we found 17 genes highly expressed in HSCL cell lines associated with HSC quiescence (such as NupR1). GSEA analyses confirmed an enrichment of several cell cycle genes as well as genes involved in unfolded protein response (UPR) and autophagy (p <0.001). Thus, we report here for the first time, a unique BCR-ABL inducible ESC-derived hematopoiesis model which demonstrates the ability of BCR-ABL expression to induce a proliferative signal for extended periods of time in highly primitive hematopoietic cells. This novel tool can be used to dissect CML ontogeny both in vitro and in vivo and perform drug screening at the most primitive stem cell level. It can also be of major interest to uncover molecular events and signaling pathways activated during normal ESC-derived hematopoiesis. Such knowledge will open up new avenues to differentiate human iPSC towards bona fide HSCs for potential clinical applications.
Figure 1. Establishment of ES cell-derived hematopoietic cell lines expressing BCR::ABLp210.(A) Representation of the timeline of ES cell differentiation protocol and doxycycline induction. (B) HSCL#A cell morphology. (C) MGG staining of HSCL#A cells. (D) Proliferation curves of HSCL#A and HSCL#C cell lines over 40 days in culture. (E)Kinetics of the effect of doxycycline removal on HSCL#A cells cultured on OP9 cells for 4 consecutive days.
Disclosures
Turhan:Novartis: Consultancy, Honoraria; Incyte Biosciences: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal